Outcomes of the DUPLEX Trial in Patients With Genetic Focal Segmental Glomerulosclerosis (gFSGS)
2024
Background
To investigate the impact of sparsentan 800 mg/day vs irbesartan 300 mg/day on partial remission (urine protein-creatinine ratio [UPCR] ≤1.5 g/g and >40% reduction from baseline) or complete remission (CR) (<0.3 g/g) of proteinuria and the effect of achieving these targets on progression to kidney failure in DUPLEX1
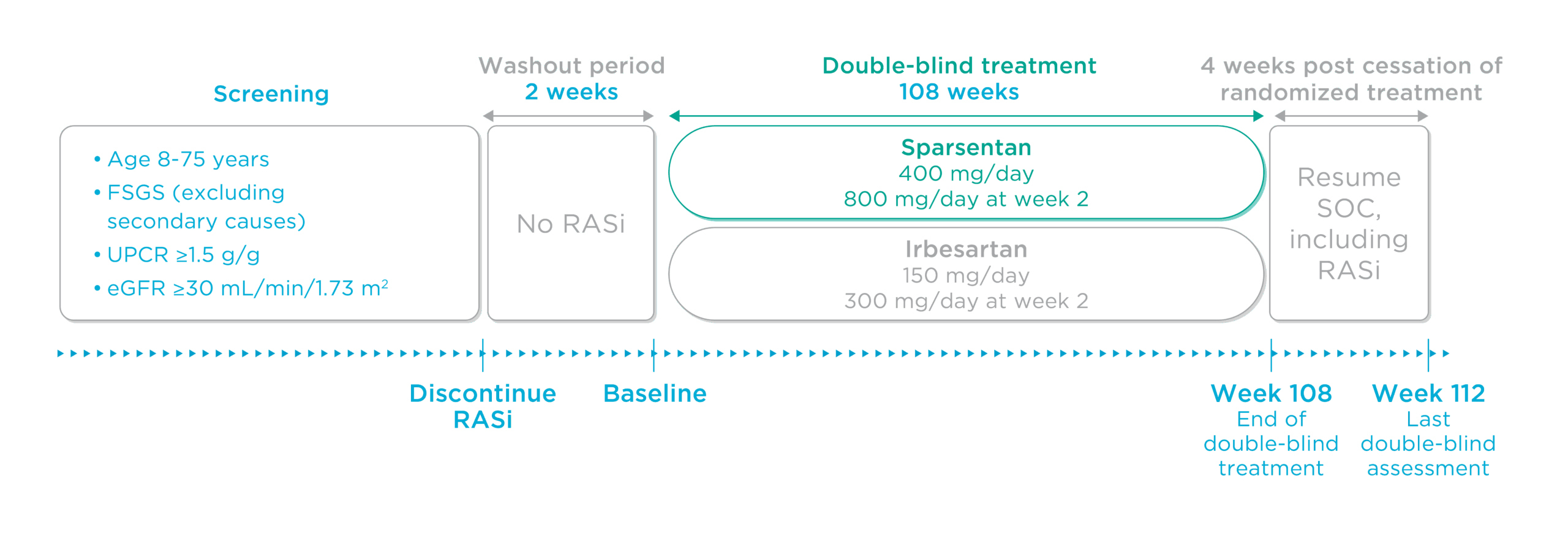
Figure. DUPLEX study design
Analyses by treatment arm evaluated the proportion of patients achieving partial remission or CR of proteinuria at any time through 108 weeks
Pooled analyses using data from both treatment arms evaluated rates of progression to kidney failure in patients who achieved vs those who did not achieve low proteinuria
Patients achieved partial remission of proteinuria earlier and more often with sparsentan vs maximum labeled dose irbesartan1
Findings demonstrated a 1.5-fold higher rate of partial remission with sparsentan vs maximum labeled dose irbesartan over 108 weeks1
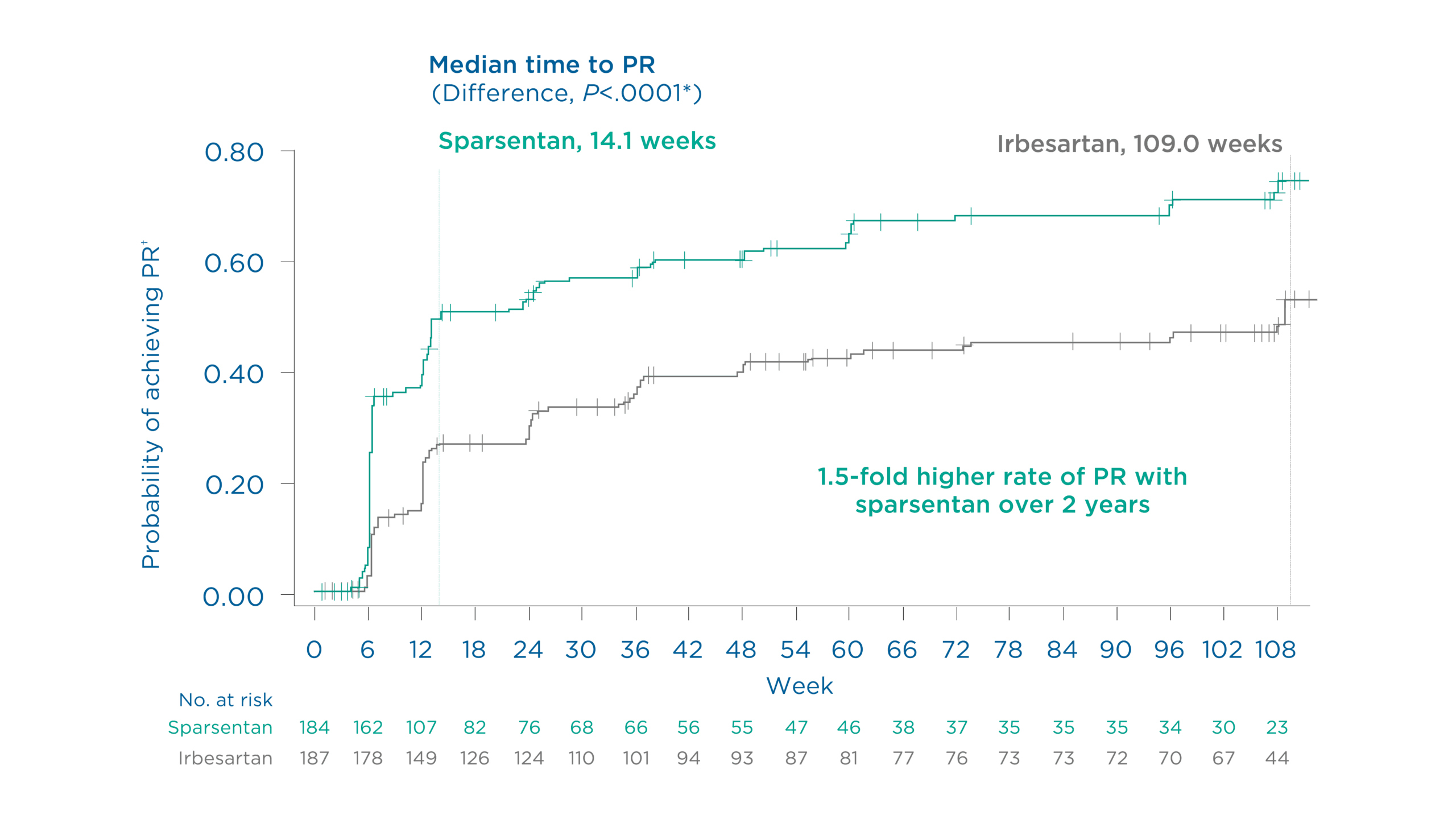
Figure. Probability of achieving partial remission† of proteinuria through 108 weeks1
The rate of partial remission† of proteinuria at 108 weeks was 64.7% (119/184) with sparsentan vs. 43.9% (82/187) with irbesartan (Relative risk: 1.48, 95% CI: 1.23 to 1.78)1
*P value generated from a stratified Cox proportional hazards model with treatment and baseline log (UPCR) as covariates, stratified by randomization stratification factors1
†UPCR ≤1.5 g/g and >40% reduction from baseline (also FSGS partial remission endpoint [FPRE])1
Patients achieved CR of proteinuria earlier and more often with sparsentan vs maximum labeled dose irbesartan1
Findings demonstrated a 2.5-fold higher rate of complete remission with sparsentan vs maximum labeled dose irbesartan over 108 weeks1
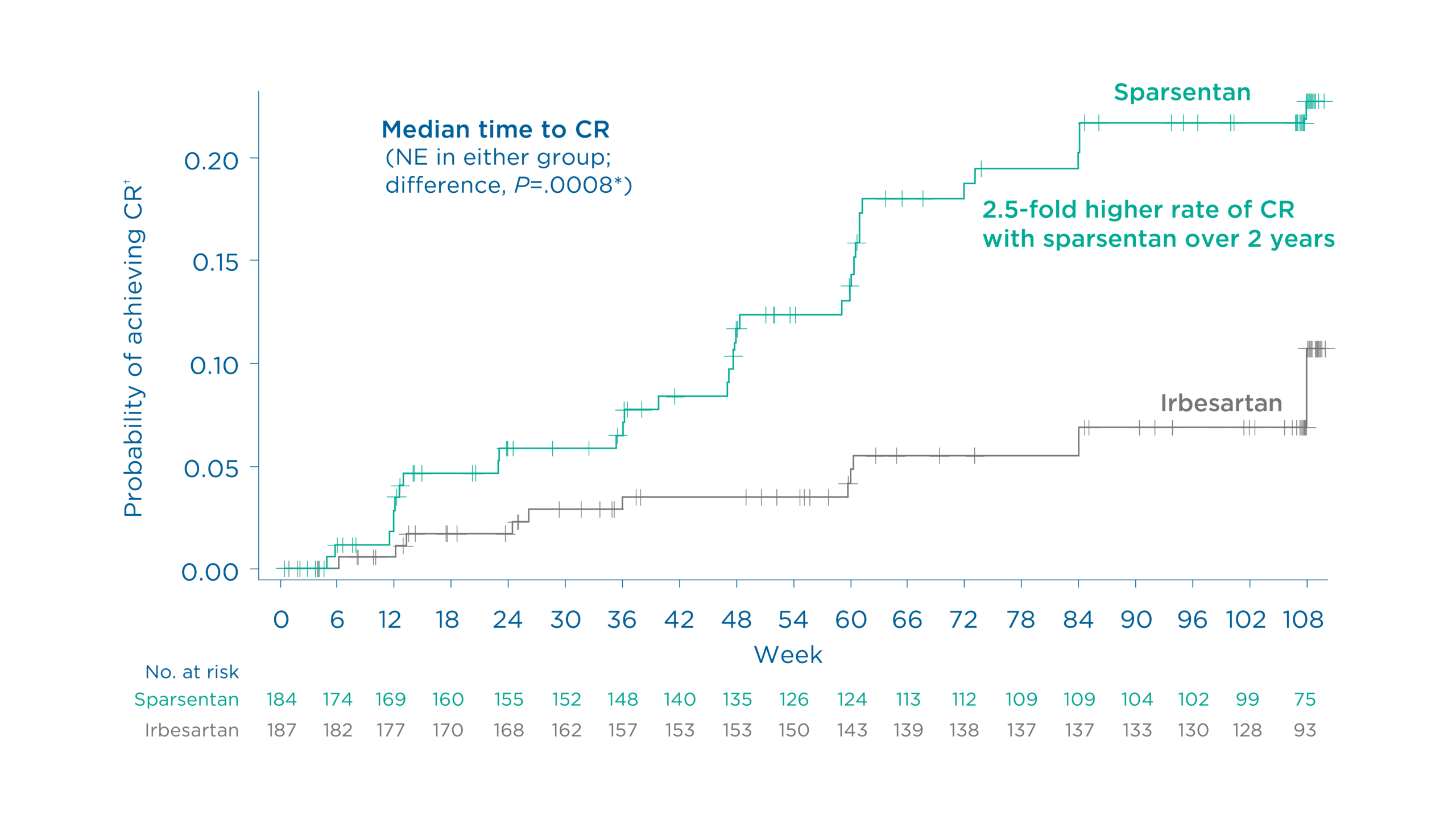
Figure. Probability of achieving complete remission† of proteinuria through 108 weeks1
The rate of CR of proteinuria at 108 weeks was 18.5% (34/184) with sparsentan vs 7.5% (14/187) with irbesartan (Relative risk: 2.47, 95% CI: 1.37 to 4.45)1
*P value generated from a stratified Cox proportional hazards model with treatment and baseline log (UPCR) as covariates, stratified by randomization stratification factors1
†UPCR <0.3 g/g1
Read more about sparsentan achieving proteinuria reduction across several low proteinuria thresholds in a DUPLEX post hoc analysis
Patients who achieved low proteinuria were less likely to reach kidney failure vs those who did not, irrespective of treatment arm1
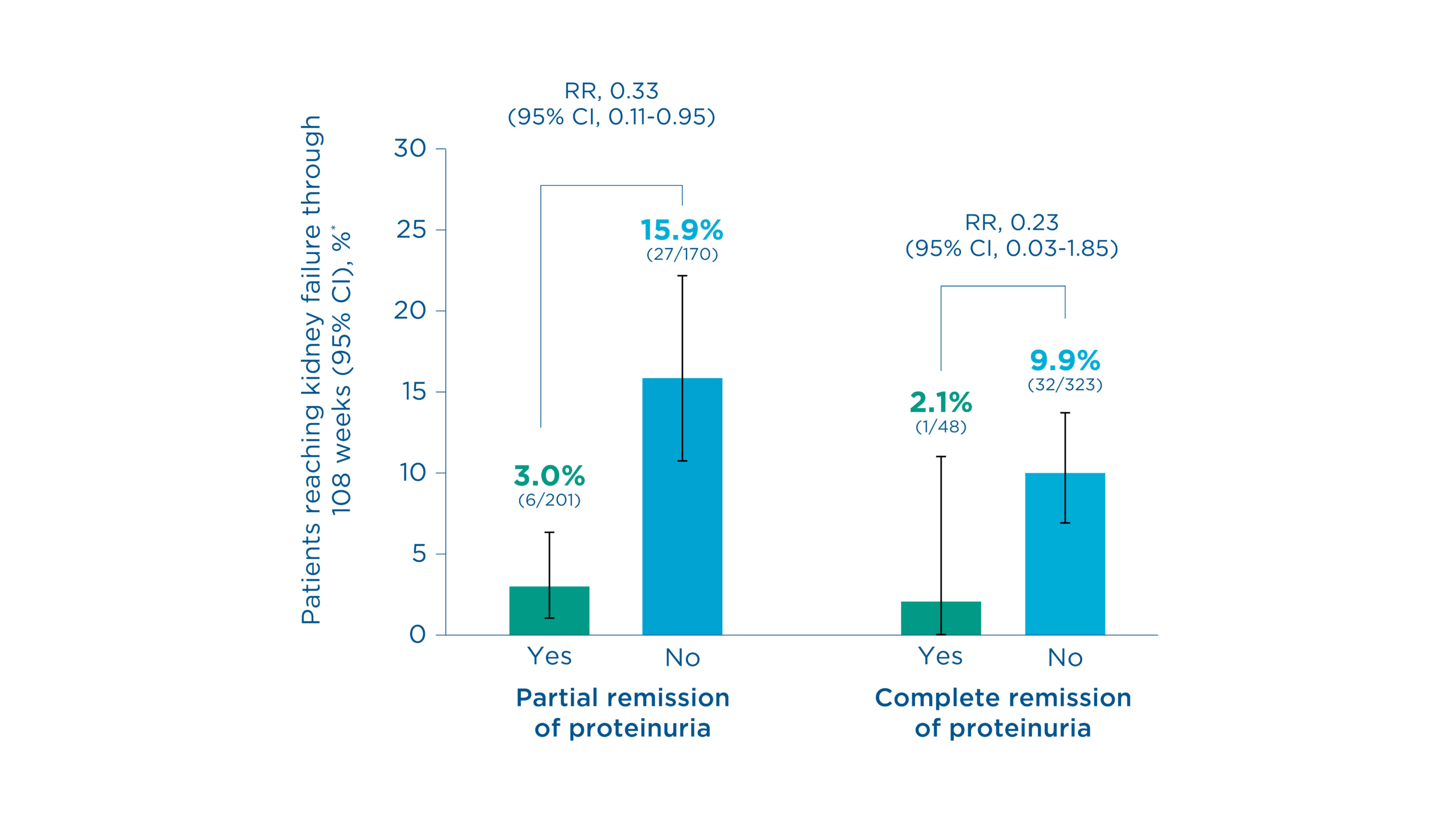
Figure. Probability of reaching kidney failure through 108 weeks†1
Kidney failure occurred in 3.0% (6/201) of patients who achieved partial remission‡ of proteinuria vs 15.9% (27/170) of those who did not (Relative risk: 0.33, 95% CI: 0.11 to 0.95)1
Kidney failure occurred in 2.1% (1/48) of patients who achieved CR§ of proteinuria vs 9.9% (32/323) of those who did not (Relative risk: 0.23, 95% CI: 0.03 to 1.85)1
*Confirmed estimated glomerular filtration rate (eGFR) of <15 mL/min/1.73 m2 or kidney replacement therapy1
†Results from post hoc analyses using pooled data irrespective of treatment arm1
‡UPCR ≤1.5 g/g and >40% reduction from baseline (also FPRE)1
§UPCR <0.3 g/g1
Sparsentan 800 mg/day was well tolerated with a safety profile comparable to that of irbesartan1
The most common treatment emergent adverse events (TEAEs) (≥15% of patients in any group) included1:
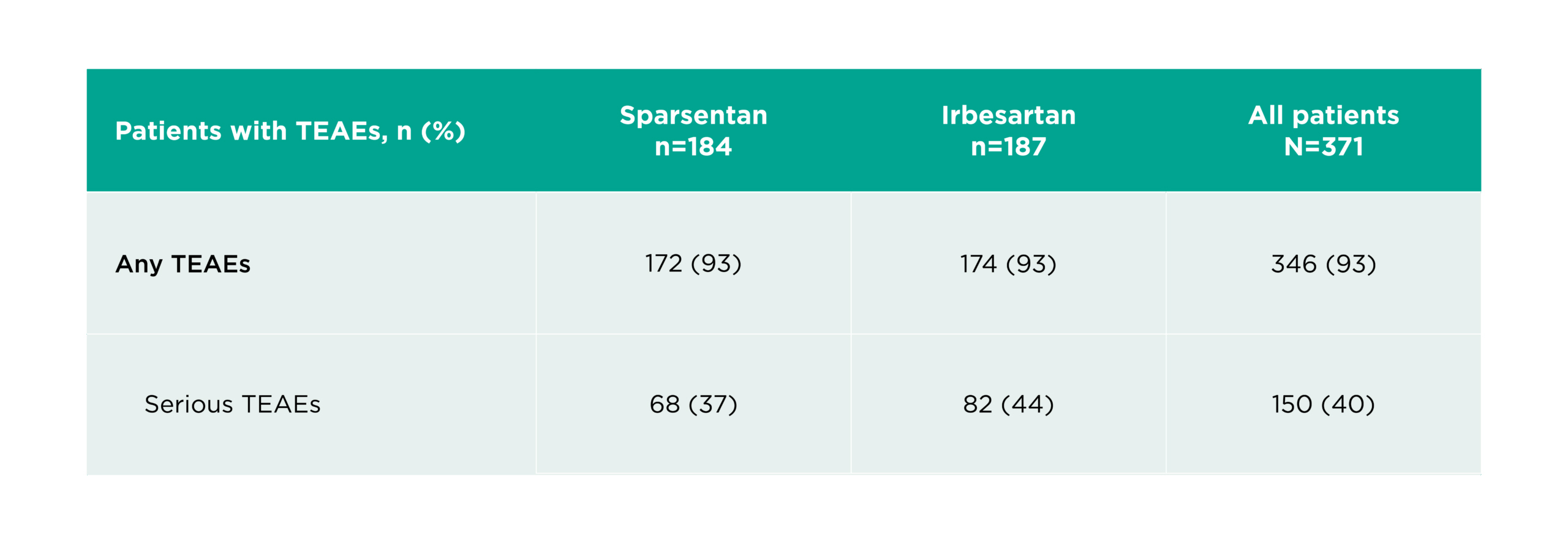
Table. Adverse events1
TEAEs were experienced by 93% (172/184) of patients with sparsentan and 93% (174/187) of patients with irbesartan1
Serious TEAEs were experienced by 37% (68/184) of patients with sparsentan and 44% (82/187) of patients with irbesartan1
In the DUPLEX study, patients with FSGS experienced rapid and sustained reduction in proteinuria with sparsentan compared to maximum labeled dose irbesartan1
Partial and complete remission was achieved earlier and more often with sparsentan1
Those who experienced low proteinuria had a lower risk of kidney failure1
Sparsentan 800 mg/day was generally well tolerated over 108 weeks of treatment1
Sparsentan is not FDA approved for the treatment of FSGS.
*The DUPLEX Study was conducted during the COVID-19 pandemic.
CI, confidence interval; COVID-19, coronavirus disease of 2019; DEARA, Dual Endothelin Angiotensin Receptor Antagonist; eGFR, estimated glomerular filtration rate; FPRE, FSGS partial remission endpoint; FSGS, focal segmental glomerulosclerosis; TEAE, treatment-emergent adverse event; UPCR, urine protein-creatinine ratio.
MA-SP-25-0054 | July 2025