Implications of Proteinuria Remission on Estimated Glomerular Filtration Rate Trajectory in Patients With IgA Nephropathy in PROTECT
2025
Background

To determine the safety, efficacy, and mechanistic action of sparsentan as first-line therapy in patients newly diagnosed with IgA nephropathy1*
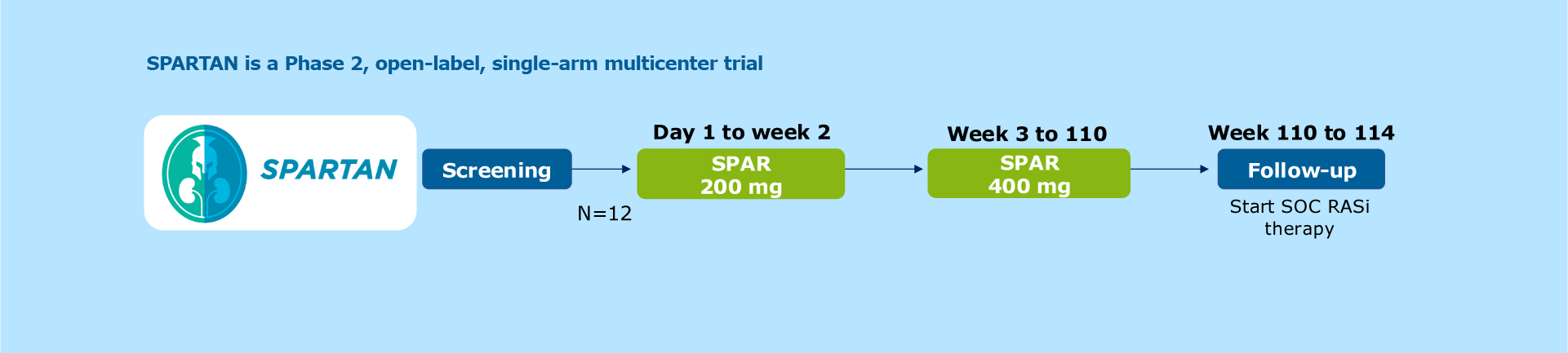
Figure. Study design
SPARTAN is a Phase 2, open-label, single-arm multicenter trial1
Patients receive 200 mg of sparsentan once daily from Day 1 to Week 2, followed by 400 mg once daily from Weeks 3 to 1101
The SPARTAN study is being conducted at 5 participating sites in the UK1
Twelve newly diagnosed treatment-naïve patients with IgA nephropathy participated in the study1
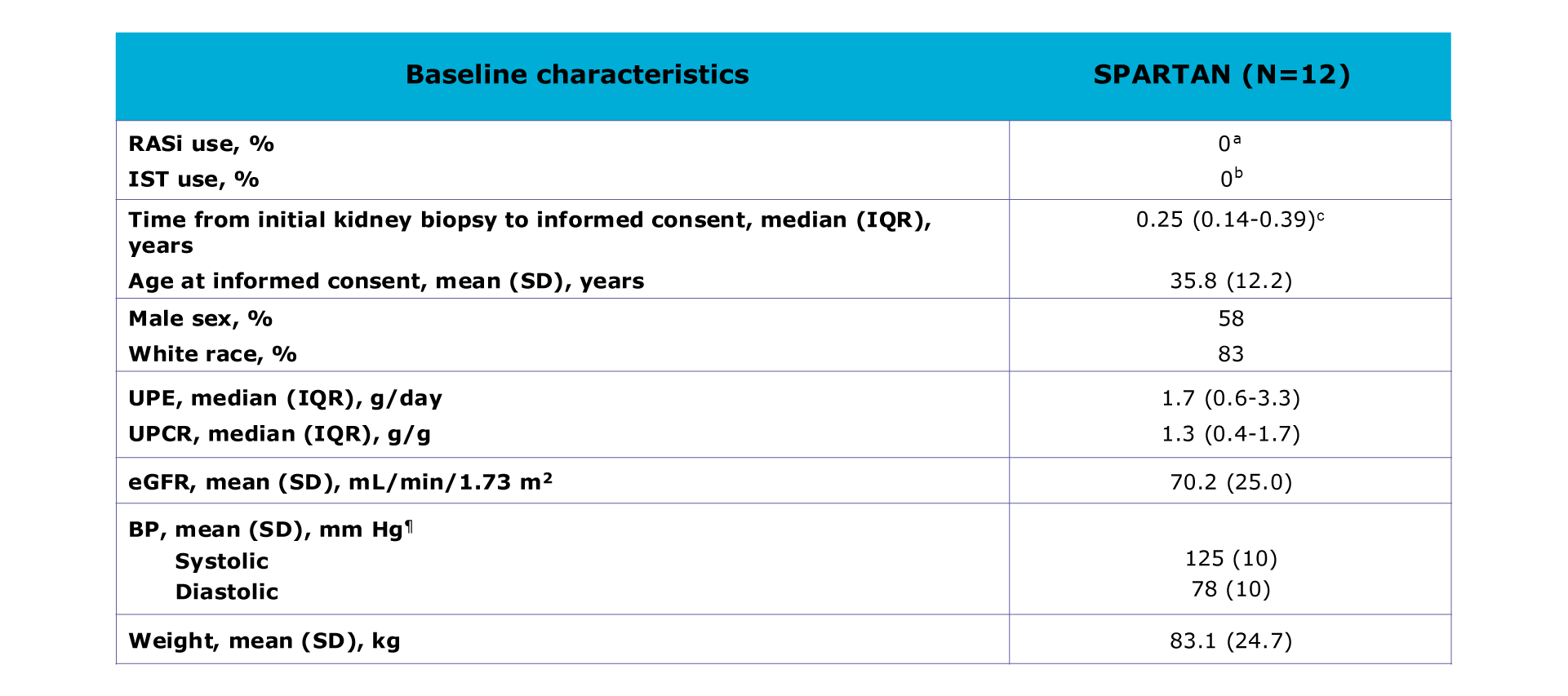
Table. Patient demographics and baseline characteristics
Patients were primarily white (83%; 10/12), male (58%, 7/12) adults with a mean age of 35.8 years at informed consent. The median time from initial kidney biopsy to informed consent was 0.25 years (IQR: 0.14-0.39)1
aEligibility criteria for SPARTAN did not allow ACEis/ARBs use within ≤12 months.1 bEligibility criteria for SPARTAN did not allow systemic IST within ≤6 months.1 cn=11.1
At baseline, patients were not on a RASi or on an IST1
Median urine protein excretion (UPE) was 1.7 g/day and median protein-creatinine ratio (UPCR) was 1.3 g/g. Mean estimated glomerular filtration rate (eGFR) was 70.2 mL/min/1.73 m2
Mean BP was 125/78 mmHg and the mean weight was 83.1 kg1
Proteinuria reductions were rapid and sustained over 24 weeks of sparsentan treatment1
Findings demonstrated1:
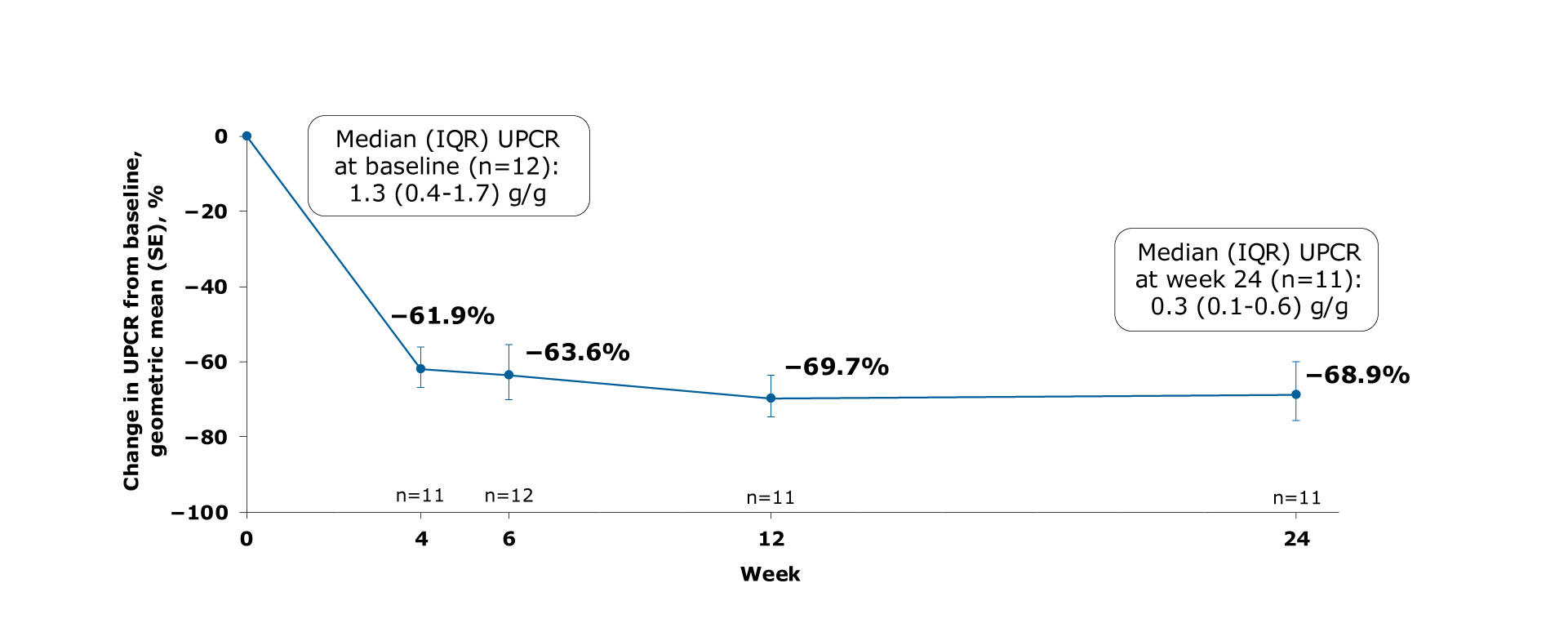
Figure. Geometric mean change from baseline in UPCR
Mean UPCR decreased from 1.3 g/g (IQR: 0.4-1.7) at baseline to 0.3 g/g (IQR: 0.1-0.6) at Week 24 of sparsentan treatment1
Mean decreases in UPCR were 61.9%, 63.6%, 69.7% and 68.9% from baseline to Weeks 4, 6, 12, and 24, respectively1
Complete remission of proteinuria (<0.3 g/d) was achieved by 58% (7/12) of patients at any time during the 24-week treatment period1
Proteinuria reductions of ≥75% at any time during the first 24 weeks of treatment were observed in 4 of 5 patients who had protein excretion of >2 g/day at baseline1
eGFR levels remained relatively stable1
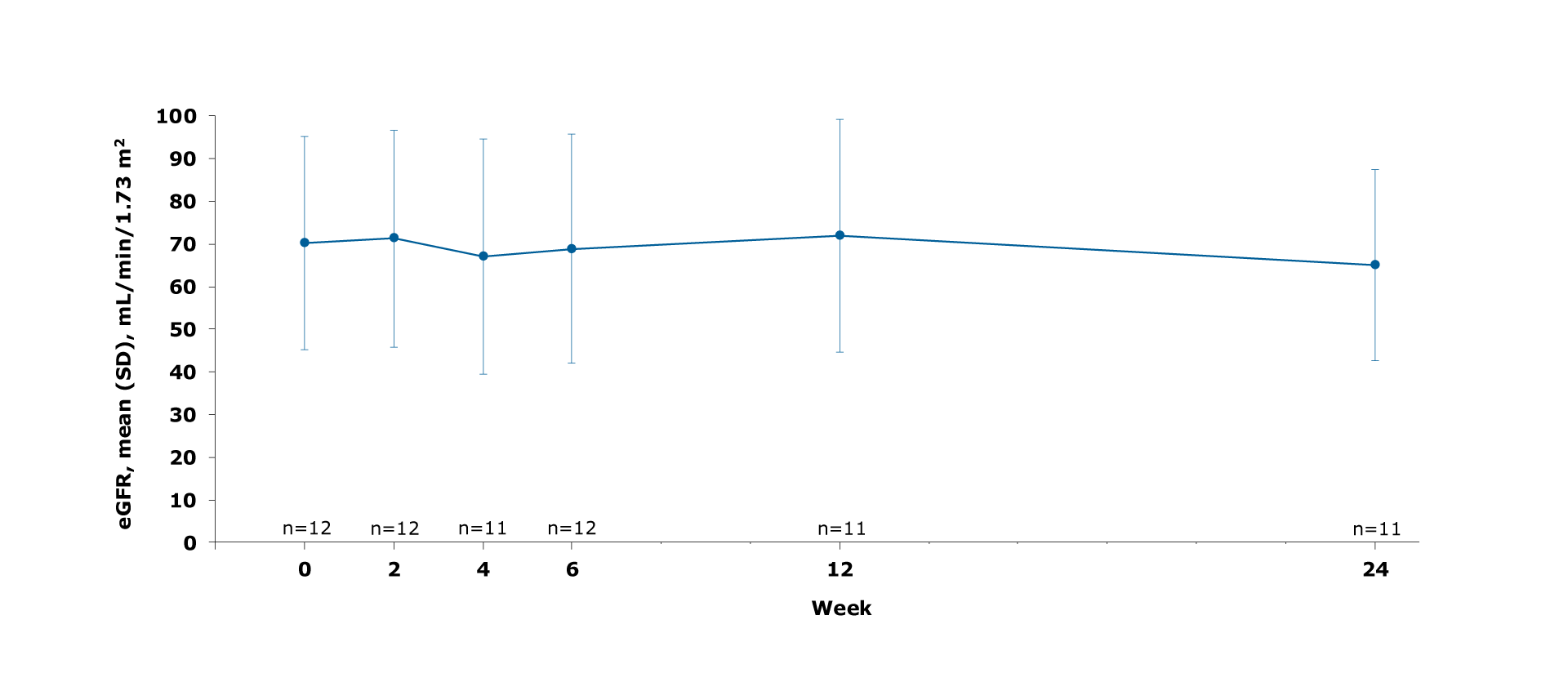
Figure. Mean eGFR change from baseline to Week 24
eGFR levels remained relatively stable over 24 weeks1
Sparsentan was generally well tolerated over 24 weeks of treatment1
One patient permanently discontinued treatment due to hypotension after Week 61
There was no evidence of fluid retention1
The most common adverse events (AE) (>2 patients) were1:
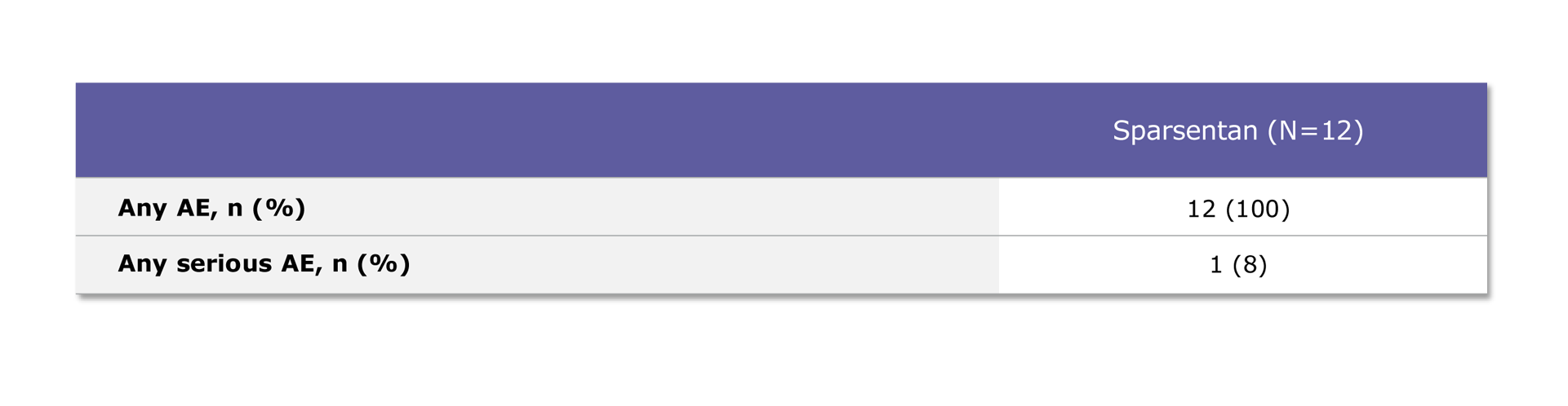
Table. Patients with any AE
Safety results are based on all patient data. All patients experienced at least one AE1
One patient experienced a serious AE, which was a limb abscess1
Urinary sCD163 (u-sCD163) is a biomarker for alternatively activated macrophages1,6
It has been correlated with kidney macrophage infiltration and active lesions in IgA nephropathy1,6
In the TESTING study, a ≥50% reduction of u-sCD163 from baseline was associated with a reduced risk of the composite kidney endpoints1,6
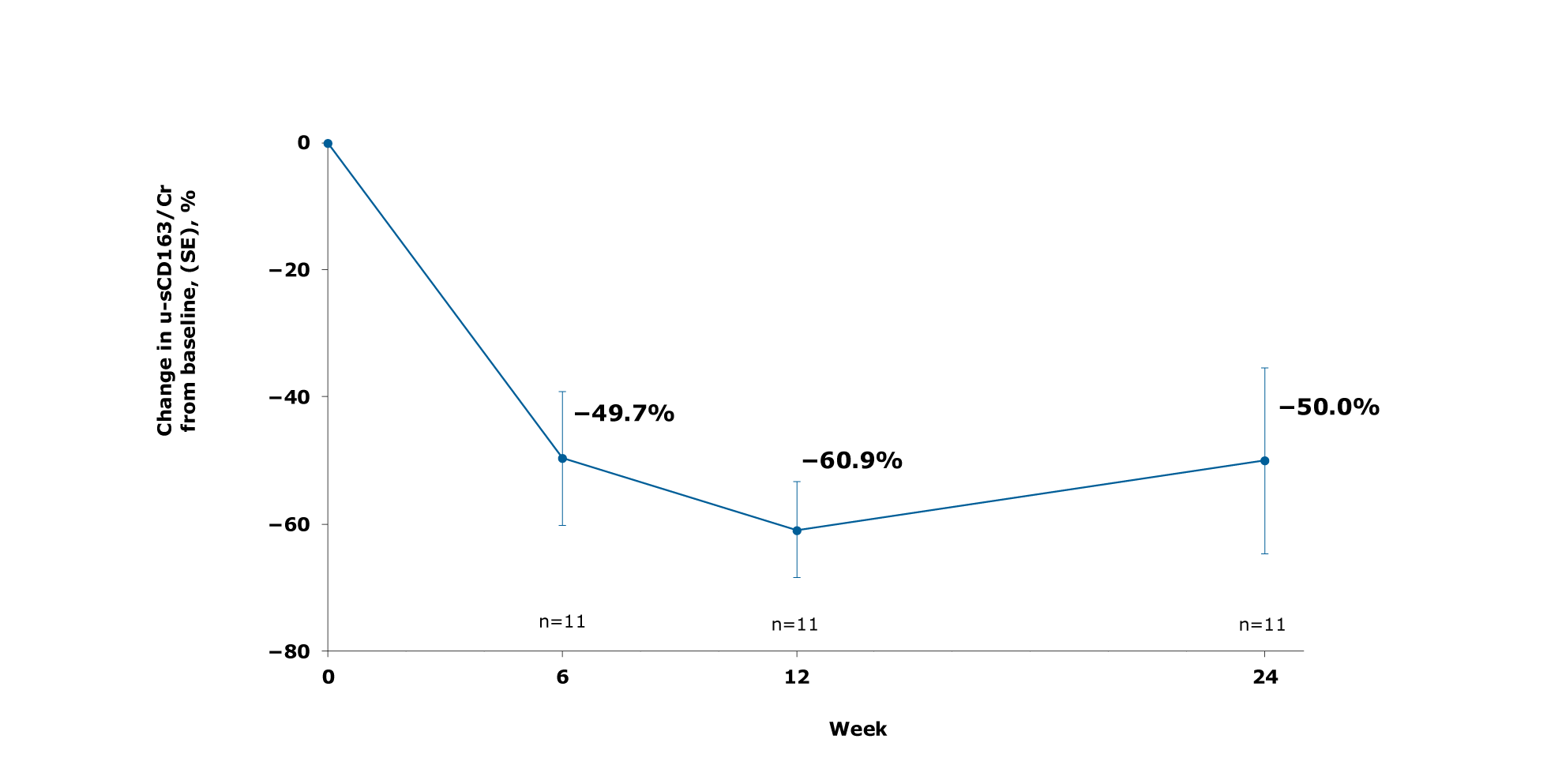
Table. u-sCD163 levels with sparsentan
Rapid and sustained reduction levels of u-sCD163 were observed with sparsentan1
Conclusions
In this Phase 2 open-label, single-arm trial, first-line treatment with sparsentan in patients newly diagnosed with IgA nephropathy led to rapid and sustained reductions in proteinuria over 24 weeks of treatment and was generally well tolerated1

*The data cutoff for this interim analysis was February 15, 2024.
ACEi, angiotensin-converting enzyme inhibitor; AE, adverse event; ARB, angiotensin receptor blocker; DEARA, Dual Endothelin Angiotensin Receptor Antagonist; eGFR, estimated glomerular filtration rate; IgA, immunoglobulin A; IQR, interquartile range; IST, immunosuppressive therapy; KDIGO, Kidney Disease Improving Global Outcomes; RASi, renin-angiotensin system inhibitor; SD, standard deviation; UPCR, urine protein-creatinine ratio; UPE, urine protein excretion; u-sCD163, urinary sCD163.
MA-SP-24-0137 | November 2024