Patient-Reported Outcomes in the PROTECT Clinical Trial Comparing Sparsentan With Irbesartan for Immunoglobulin A Nephropathy
2024
Background

To determine the treatment effects of sparsentan versus the maximum labeled dose irbesartan in patients with IgA nephropathy between patients subgroups with baseline urine protein-creatinine ratio (UPCR) <1.0 and ≥1.0 g/g1

Figure. PROTECT study design
PROTECT is a large, international, randomized, double-blind, active-controlled Phase 3 trial to assess the efficacy and safety of sparsentan versus irbesartan. This analysis evaluates subgroups of UPCR <1 versus ≥1 g/g at baseline1
a95% and 97% of patients titrated to maximum labeled dose of sparsentan and irbesartan, respectively.1
Participants were randomly assigned to the treatment, sparsentan or active-control, maximum labeled dose irbesartan group1
Study included a 110-week double-blind treatment period and 4 weeks of no treatment. Interim analysis occurred at 36 weeks. Two-year follow-up occurred at 110 weeks. There was an inclusion criterion of urinary protein excretion (UPE) ≥1 g/day at screening1
Patient demographics and baseline characteristics were well balanced between treatment arms1
Sparsentan demonstrated superior rapid and sustained reductions in UPCR regardless of baseline UPCR levels versus maximum labeled dose irbesartan1
Similarly, complete remission (CR) of proteinuria was achieved earlier and more frequently in patients treated with sparsentan versus maximum labeled dose irbesartan regardless of baseline UPCR level1
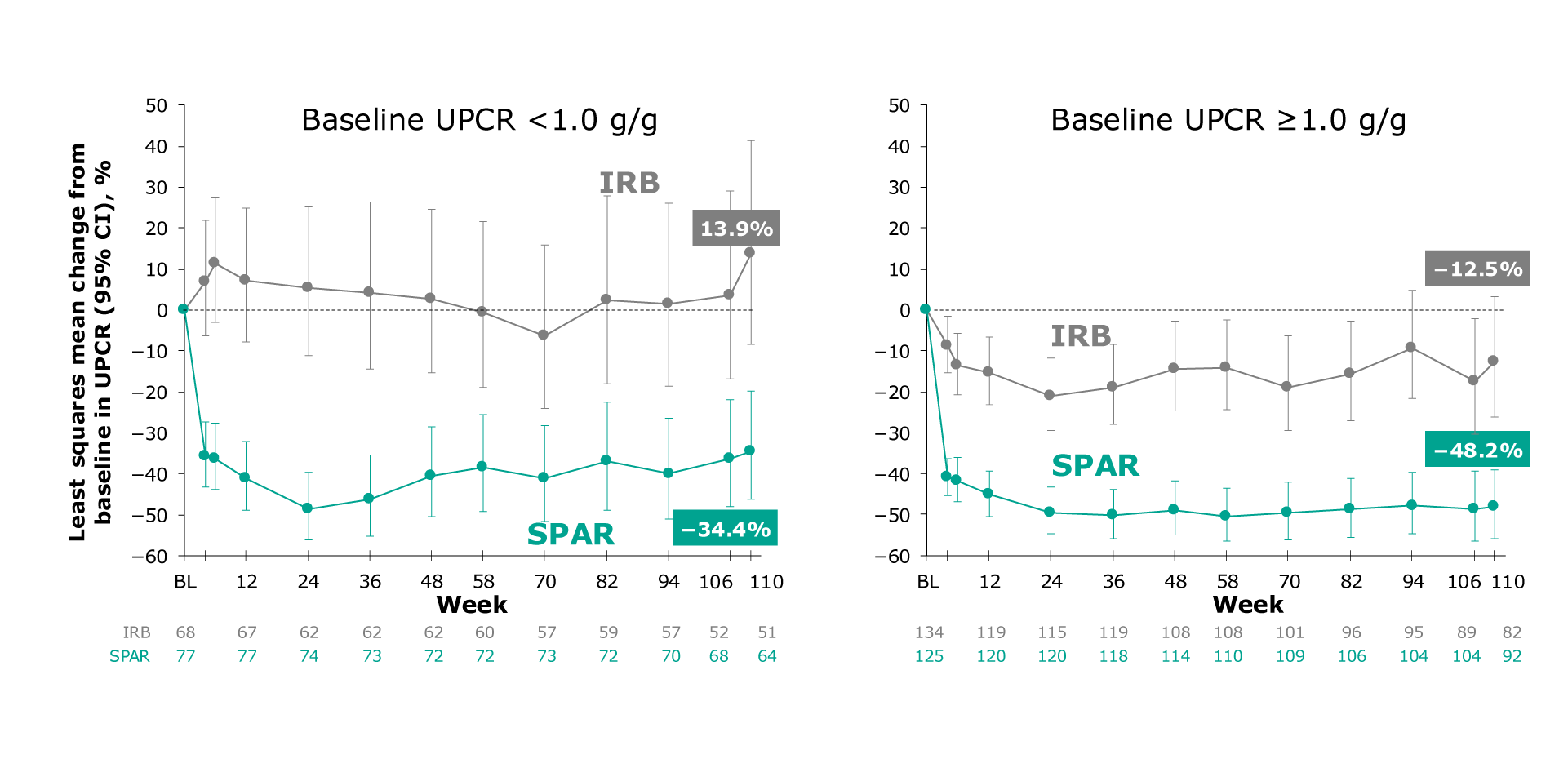
Figure. LS mean change from baseline in UPCR for the population with UPCR <1 g/g and UPCR ≥1 g/g
At Week 110, patients with a baseline UPCR <1.0 g/g showed a 34.4% reduction when treated with sparsentan versus a 13.9% increase with maximum labeled dose irbesartan1
At Week 110, patients with a baseline UPCR ≥1.0 g/g showed a 48.2% reduction when treated with sparsentan versus a 12.5% reduction with maximum labeled dose irbesartan1
In patients with baseline UPCR <1.0 g/g, sparsentan led to a 42% relative reduction versus maximum labeled dose irbesartan with a ratio of 0.58 (95% CI: 0.43 to 0.77)1
In patients with baseline UPCR ≥1.0 g/g, sparsentan led to a 41% relative reduction versus maximum labeled dose irbesartan with a ratio of 0.59 (95% CI: 0.47 to 0.75)1
Greater kidney function preservation with sparsentan was consistently seen regardless of baseline UPCR level1
Absolute change in estimated glomerular filtration rate (eGFR) from baseline to Week 110 was lower with sparsentan versus maximum labeled irbesartan1
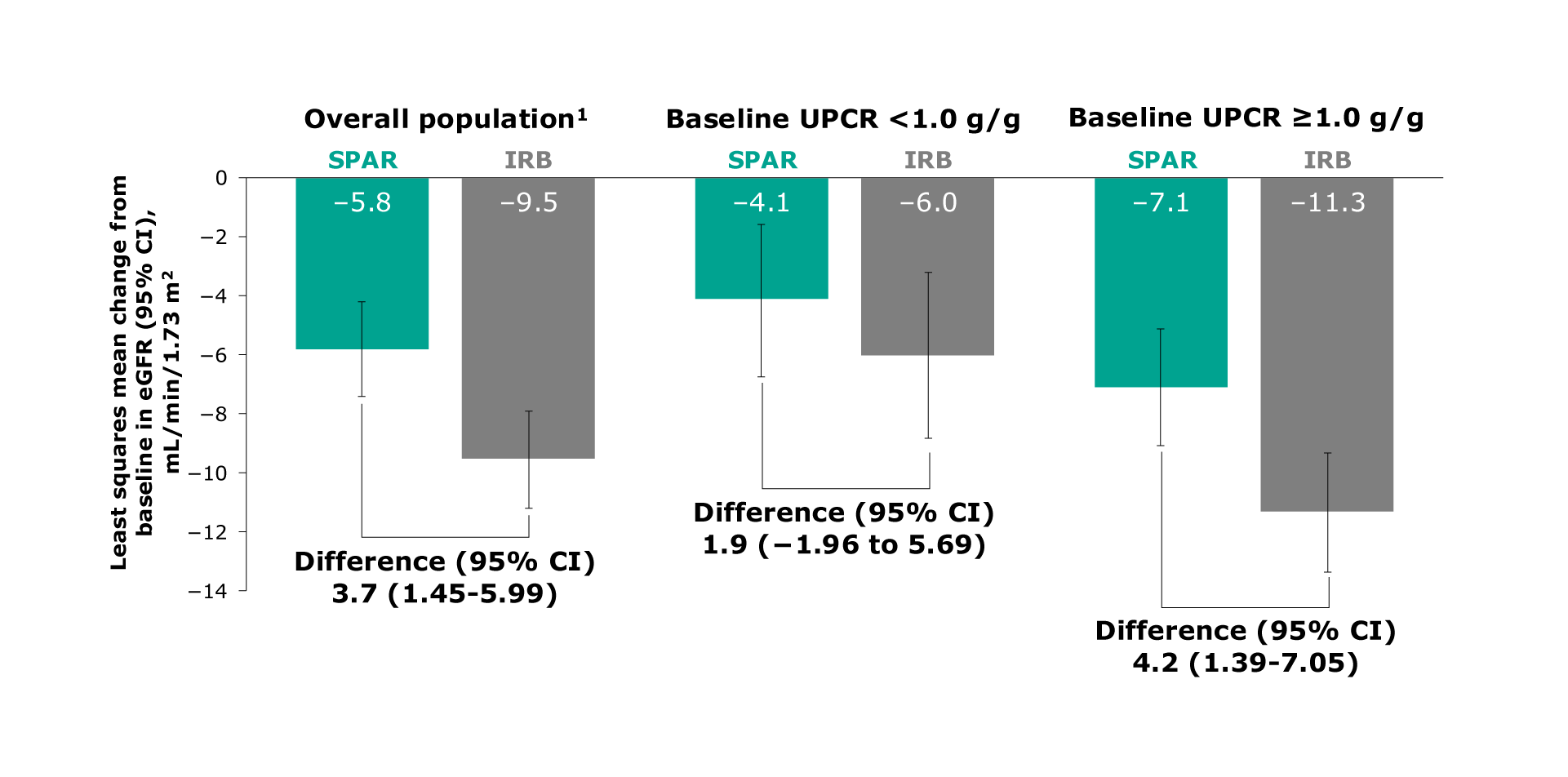
Figure. LS mean change from baseline in eGFR for the overall study, UPCR <1 g/g, and UPCR ≥1 g/g populations
In the overall population, sparsentan demonstrated a difference of 3.7 mL/min per 1.73 m2. In the population with baseline UPCR <1.0 g/g, sparsentan demonstrated a difference of 1.9 mL/min per 1.73 m2. In the population with baseline UPCR ≥1.0 g/g, sparsentan demonstrated a difference of 4.2 mL/min per 1.73 m2
In the overall population, sparsentan demonstrated a -5.8 mL/min per 1.73 m2 decrease in eGFR versus a -9.5 mL/min per 1.73 m2 decrease with maximum labeled dose irbesartan (Difference: 3.7, 95% CI: 1.45 to 5.99)1
In the population with baseline UPCR <1.0 g/g, sparsentan demonstrated a -4.1 decrease in eGFR versus a -6.0 mL/min per 1.73 m2 decrease with maximum labeled dose irbesartan (Difference: 1.9, 95% CI: 1.96 to 5.69)1
In the population with baseline UPCR ≥1.0 g/g, sparsentan demonstrated a -7.1 mL/min per 1.73 m2 decrease in eGFR versus a -11.3 mL/min per 1.73 m2 decrease with maximum labeled dose irbesartan (Difference: 4.2, 95% CI: 1.39 to 7.05)1
Sparsentan was well tolerated with a consistent safety profile comparable to maximum labeled dose irbesartan across baseline proteinuria subgroups1
The most common adverse events (AEs) [≥15% of patients in any group] were:
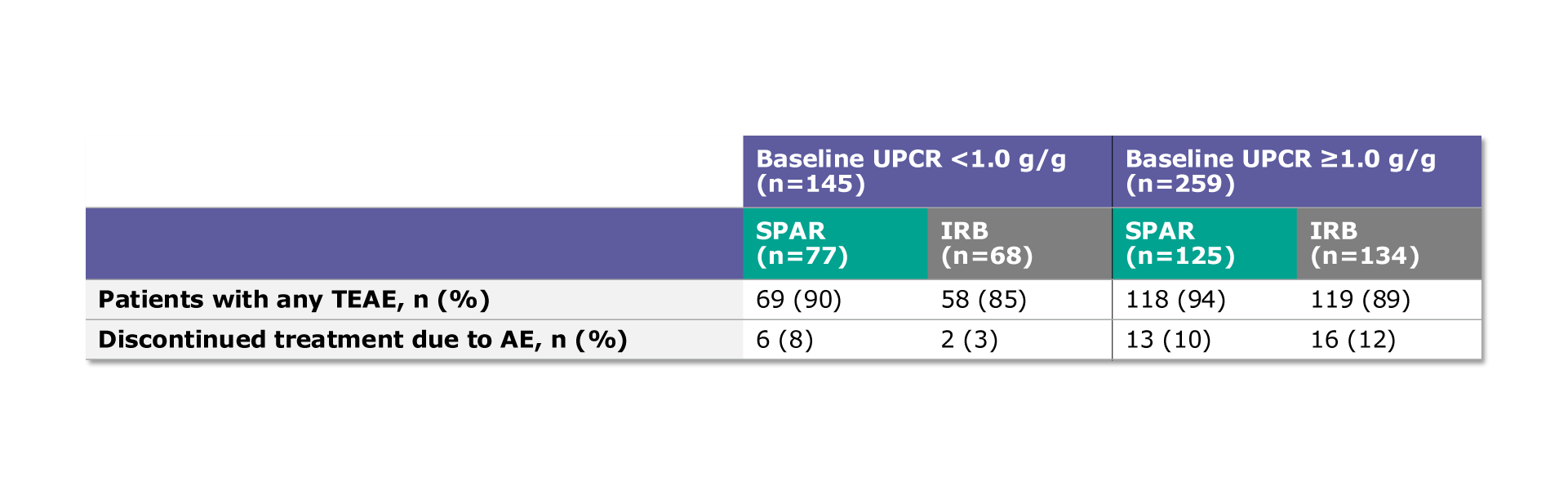
Table. Treatment-emergent adverse events (TEAE) and treatment discontinuations due to an AE
In the population with baseline UPCR <1.0 g/g, 90% (69/77) of patients on sparsentan and 85% (58/68) of patients on maximum labeled dose irbesartan experienced a TEAE1
In the population with baseline UPCR ≥1.0 g/g, 94% (118/125) of patients on sparsentan and 89% (119/134) of patients on maximum labeled dose irbesartan experienced a TEAE1
In the population with baseline UPCR <1.0 g/g, 8% (6/77) of patients on sparsentan and 3% (2/68) of patients on maximum labeled dose irbesartan discontinued treatment due to an AE1
In the population with baseline UPCR ≥1.0 g/g, 10% (13/125) of patients on sparsentan and 12% (16/134) of patients on maximum labeled dose irbesartan discontinued treatment due to an AE1
Conclusions
Sparsentan has demonstrated nephroprotective treatment effects that are consistent across baseline proteinuria levels in patients with IgA nephropathy1
Regardless of baseline UPCR level, when compared to maximum labeled dose irbesartan, sparsentan1:
PROTECT trial was conducted during the COVID-19 pandemic.
AE, adverse event; CI, confidence interval; COVID-19, coronavirus disease 2019; DEARA, Dual Endothelin Angiotensin Receptor Antagonist; eGFR, estimated glomerular filtration rate; IgA, immunoglobulin A; IRB, irbesartan; KDIGO, Kidney Disease Improving Global Outcomes; LS, least square; SPAR, sparsentan; TEAE, treatment-emergent adverse event; UPCR, urine protein-creatinine ratio;
UPE, urinary protein excretion.
MA-SP-24-0133 | November 2024